Abstract
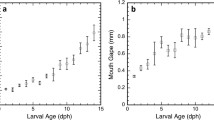
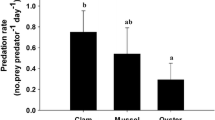
We quantified feeding rates of field caught Aurelia aurita feeding on yolk sac cod (Gadus morhua) larvae in a series of incubation experiments. A short-time (~1 h) functional response experiment with a wide range of prey concentrations (0.5–16 prey l−1, initial concentration) revealed that ingestion rates increased linearly over this range, such that clearance rates were similar between the different prey concentrations. This suggests that A. aurita is capable of efficiently utilizing dense prey patches. This indication was further supported by a linear increase of prey captured by A. aurita during 2.5 h of feeding at extremely high prey concentration (>200 prey l−1). Clearance rate in darkness scaled with jellyfish diameter to a power of ~1.7 for jellyfish 3.9–9.5 cm in diameter. The jellyfish did not alter their umbrella pulse frequency in response to presence of fish larvae. There were no significant differences between A. aurita feeding rates in light and darkness for yolk sac prey ages 0–7 days (at 7.5°C). Although prey vision and escape abilities of fish may develop rapidly during early larval ontogeny, these factors apparently have little impact on interactions with predators such as A. aurita during the yolk sac stage.








Similar content being viewed by others
References
Aksnes DL, Nejstgaard J, Soedberg E, Sørnes T (2004) Optical control of fish and zooplankton populations. Limnol Oceanogr 49 (1):233–238
Arai MN, Hay DE (1982) Predation by medusae on Pacific herring (Clupea harengus Pallasi) larvae. Can J Fish Aquat Sci 39:1537–1540
Bailey KM (1984) Comparison of laboratory rates of predation on five species of marine fish larvae by three planktonic invertebrates—effects of larval size on vulnerability. Mar Biol 79:303–309
Bailey KM, Batty RS (1983) A laboratory study of predation by Aurelia aurita on larval herring (Clupea harengus): experimental observations compared with model predictions. Mar Biol 72:295–301
Bailey KM, Batty RS (1984) Laboratory study of predation by Aurelia aurita on larvae of cod, flounder, plaice and herring—development and vulnerability to capture. Mar Biol 83:287–291
Blaxter JHS (1968) Visual thresholds and spectral sensitivity of herring larvae. J Exp Biol 48:39–53
Blaxter JHS (1986) Development of sense-organs and behavior of teleost larvae with special reference to feeding and predator avoidance. Trans Am Fish Soc 115:98–114
Blaxter JHS, Fuiman LA (1990) The role of the sensory systems of herring larvae in evading predatory fishes. J Mar Biol Assoc UK 70:413–427
Brodeur RD, Sugisaki H, Hunt GL (2002) Increases in jellyfish biomass in the Bering Sea: implications for the ecosystem. Mar Ecol Prog Ser 233:89–103
Båmstedt U (1990) Trophodynamics of the scyphomedusae Aurelia aurita—predation rate in relation to abundance, size and type of prey organism. J Plankton Res 12:215–229
Båmstedt U, Martinussen MB, Matsakis S (1994) Trophodynamics of the 2 scyphozoan jellyfishes, Aurelia-aurita and Cyanea-capillata, in western Norway. ICES J Mar Sci 51:369–382
Cobcroft JM, Pankhurst PM (2003) Sensory organ development in cultured striped trumpeter larvae Latris lineata: implications for feeding behaviour. Mar Freshwater Res 54(5):669–682
Colin SP, Costello JH (2002) Morphology, swimming performance and propulsive mode of six co-occurring hydromedusae. J Exp Biol 205:427–437
Costello JH, Colin SP (1995) Flow and feeding by swimming scyphomedusae. Mar Biol 124:399–406
Costello JH, Colin SP (1994) Morphology, fluid motion and predation by the scyphomedusa Aurelia aurita. Mar Biol 121:327–334
Costello JH, Colin SP (2002) Prey resource use by coexistent hydromedusae from Friday Harbor, Washington. Limnol Oceanogr 47:934–942
Cowan JH Jr, Birdsong RS, Houde ED, Priest JS, Sharp WC, Mateja GB (1992) Enclosure experiments on survival and growth of black drum eggs and larvae in lower Chesapeake bay. Estuaries 15:392–402
Cowan JH Jr, Houde ED (1992) Size-dependent predation on marine fish larvae by ctenophores, scyphomedusae, and planktovorous fish. Fish Oceanogr 1:113–126
Cowan JH, Houde ED (1993) Relative predation potentials of scyphomedusae, ctenophores and planktivorous fish on ichthyoplankton in Chesapeake Bay. Mar Ecol Prog Ser 95:55–65
Dabiri JO, Colin SP, Costello JH, Gharib M (2005) Flow patterns generated by oblate medusan jellyfish: field measurements and laboratory analyses. J Exp Biol 208:1257–1265
De Lafontaine Y, Legett WC (1987) Effect of container size on estimates of mortality and predation rates in experiments with macrozooplankton and larval fish. Can J Fish Aquat Sci 44:1534–1543
De Lafontaine Y, Legett WC (1988) Predation by jellyfish on larval fish: an experimental evaluation employing in situ enclosures. Can J Fish Aquat Sci 45:1173–1190
Duffy JT, Epifanio CE, Fuiman LA (1997) Mortality rates imposed by three scyphozoans on red drum (Sciaenops ocellatus Linnaeus) larvae in field enclosures. J Exp Mar Biol Ecol 212 (1):123–131
Eiane K, Aksnes DL, Bagøien E, Kaartvedt S (1999) Fish or jellies—a question of visibility? Limnol Oceanogr 44:1352–1357
Elliott JK, Leggett WC (1996) The effect of temperature on predation rates of a fish (Gasterosteus aculeatus) and a jellyfish (Aurelia aurita) on larval capelin (Mallotus villosus). Can J Fish Aquat Sci 53:1393–1402
Elliott JK, Leggett WC (1997) Influence of temperature on size-dependent predation by a fish (Gasterosteus aculeatus) and a jellyfish (Aurelia aurita) on larval capelin (Mallotus villosus). Can J Fish Aquat Sci 54:2759–2766
Fancett MS, Jenkins GP (1988) Predatory impact of scyphomedusae on ichtyoplankton and other zooplankton in Port Phillip Bay. J Exp Mar Biol Ecol 116:63–77
Ford MD, Costello JH, Heidelberg KB, Purcell JE (1997) Swimming and feeding by the scyphomedusa Chrysaora quinquecirrha. Mar Biol 129:355–362
Fuiman LA, Cowan JH (2003) Behavior and recruitment success in fish larvae: Repeatability and covariation of survival skills. Ecology 84:53–67
Gamble JC, Hay SJ (1989) Predation by the scyphomedusan Aurelia aurita on herring larvae in large enclosures: effects of predator size and prey starvation. Rapp P-v Réun Cons Int Explor Mer 191:366–375
Gatz Jr AJ, Kennedy VS, Mihursky JA (1973) Effects of temperature on activity and mortality of the scyphozoan medusa, Chrysaora quinquecirrha. Chesapeake Sci 14:171–180
Hansson LJ, Moeslund O, Kiørboe T, Riisgård HU (2005) Clearance rates of jellyfish and their potential predation impact on zooplankton and fish larvae in a neritic ecosystem (Limfjorden). Mar Ecol Prog Ser 304:117–131
Higgs DM, Fuiman LA (1996) Ontogeny of visual and mechanosensory structure and function in Atlantic menhaden Brevoortia tyrannus. J Exp Biol 199:2619–2629
Higgs DM, Fuiman LA (1998) Associations between behavioural ontogeny and habitat change in clupeoid larvae. J Mar Biol Ass UK 78 (4):1281–1294
Horridge GA (1956) The nerves and muscles of medusae V. Double innervation in Scyphozoa. J Exp Biol 33:366–383
Horstman E (1934) Untersuchungen zur Physiologie der Schwimmbewegungen der Scyphomedusan. Pflügers Archiv Ges Physiol 234:406–420
Kremer P (1979) Predation by the ctenophore Mnemiopsis leidyi in Narragansett Bay, Rhode-Island. Estuaries 2:97–105
Mackie GO, Larson RJ, Larson KS (1981) Swimming and vertical migration of Aurelia aurita in a deep tank. Mar Behav Physiol 7:321–329
Martinussen MB, Båmstedt U (1995) Diet, estimated daily food ration and predator impact by the scyphozoan jellyfishes Aurelia aurita and Cyanea capillata. In: Skjoldal HR, Hopkins C, Erikstad KE, Leinaas HP (eds) Ecology of fjords and coastal waters. Elsevier, Amsterdam, pp 127–145
Martinussen MB, Båmstedt (2001) Digestion rate in relation to temperature of two gelatinous planktonic predators. Sarsia 86:21–35
McHenry MJ, Jed J (2003) The ontogenetic scaling of hydrodynamics and swimming performance in jellyfish (Aurelia aurita). J Exp Biol 206:4125–4137
Munk P, Christensen V, Paulsen H (1986) Studies of a larval herring (Clupea harengus L.) patch in the Buchan area. II. Growth, mortality and drift of larvae. Dana 6:11–24
Munk P, Larsson PO, Danielssen DS, Moksness E (1999) Variability in frontal zone formation and distribution of gadoid fish larvae at the shelf break in the northeastern North Sea. Mar Ecol Prog Ser 177:221–233
Munk P, Wright PJ, Pihl NJ (2002) Distribution of the early larval stages of cod, plaice and lesser sandeel across haline fronts in the North Sea. Estuar Coast Shelf Sci 55:139–149
Möller H (1980) Scyphomedusae as predators and food competitors of larval fish. Meeresforschung 28:90–100
Möller H (1984) Reduction of a larval herring population by a jellyfish predator. Science 224:621–622
Nielsen AS, Pedersen AW, Riisgård HU (1997) Implications of density driven currents for interaction between jellyfish (Aurelia aurita) and zooplankton in a Danish fjord. Sarsia 82:297–305
Pankhurst PM, Eagar R (1996) Changes in visual morphology through life history stages of the New Zealand snapper, Pagrus auratus. New Zealand J Mar Freshw Res 30(1):79–90
Paradis AR, Pepin P, Brown JA (1996) Vulnerability of fish eggs and larvae to predation: review of the influence of the relative size of prey and predator. Can J Fish Aquat Sci 53:1226–1235
Purcell JE, Siferd TD, Marliave JB (1987) Vulnerability of larval herring (Clupea harengus pallasi) to capture by the jellyfish Aequorea victoria. Mar Biol 94:157–162
Purcell JE, Arai MN (2001) Interactions of pelagic cnidarians and ctenophores with fish: a review. Hydrobiol 451:27–44
Reeve MR, Walter MA (1978) Laboratory studies of ingestion and food utilization in lobate and tentaculate ctenophores. Limnol Oceanogr 23:740–751
Skajaa K, Fernö A, Folkvord A (2003) Swimming, feeding and predator avoidance in cod larvae (Gadus morhua L.): tradeoffs between hunger and predation risk. In: Browman HT, Skiftesvik AB (eds) The big fish Bang. Proceedings of the 26th annual larval fish conference. Institute of Marine Research, pp 105–121
Suchman CL, Sullivan BK (1998) Vulnerability of the copepod Acartia tonsa to predation by the scyphomedusa Chrysaora quinquecirrha: effect of prey size and behavior. Mar Biol 132:237–245
Suchman CL, Sullivan BK (2000) Effect of prey size on vulnerability of copepods to predation by the scyphomedusae Aurelia aurita and Cyanea sp. J Plankton Res 22:2289–2306
Stoecker DK, Michaels AE, Davis LH (1987) Grazing by the jellyfish, Aurelia-aurita, on microzooplankton. J Plankton Res 9:901–915
Sørnes TA, Aksnes DL (2004) Predation efficiency in visual and tactile zooplanktivores. Limnol Oceanogr 49 (1):69–75
Taggart CT, Leggett WC (1987) Short-term mortality in post-emergent larval capelin Mallotus villosus. I. Analysis of multiple in situ estimates. Mar Ecol Prog Ser 41:205–217
Titelman J (2001) Swimming and escape behavior of copepod nauplii: implications for predator–prey interactions among copepods. Mar Ecol Prog Ser 213:203–213
Acknowledgements
We thank Laurianne Gandon and Anne Goarant for catching jellyfish, Frank Midtøy for advice on rearing cod larvae and Erling Otterlei, SagaFjord Sea Farm AS (Stord, Norway) for providing cod eggs. Thomas Kiørboe kindly commented on an earlier draft. This work formed part of the EUROGEL project funded by the European Commission (EVK3-CT-2002–00074). The experiments comply with the current laws of Norway, where the experiments were performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Kühl, Helsingør
Rights and permissions
About this article
Cite this article
Titelman, J., Hansson, L.J. Feeding rates of the jellyfish Aurelia aurita on fish larvae. Marine Biology 149, 297–306 (2006). https://doi.org/10.1007/s00227-005-0200-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-005-0200-5